Policy Analysis
120 Years of Nanosilver History: Implications for Policy Makers
- Abstract
 Full Text HTML
Full Text HTML-
 Hi-Res PDF[4850 KB]
Hi-Res PDF[4850 KB]  PDF w/ Links[847 KB]
PDF w/ Links[847 KB]
Abstract
Nanosilver is one nanomaterial that is currently under a lot of scrutiny. Much of the discussion is based on the assumption that nanosilver is something new that has not been seen until recently and that the advances in nanotechnology opened completely new application areas for silver. However, we show in this analysis that nanosilver in the form of colloidal silver has been used for more than 100 years and has been registered as a biocidal material in the United States since 1954. Fifty-three percent of the EPA-registered biocidal silver products likely contain nanosilver. Most of these nanosilver applications are silver-impregnated water filters, algicides, and antimicrobial additives that do not claim to contain nanoparticles. Many human health standards for silver are based on an analysis of argyria occurrence (discoloration of the skin, a cosmetic condition) from the 1930s and include studies that considered nanosilver materials. The environmental standards on the other hand are based on ionic silver and may need to be re-evaluated based on recent findings that most silver in the environment, regardless of the original silver form, is present in the form of small clusters or nanoparticles. The implications of this analysis for policy of nanosilver is that it would be a mistake for regulators to ignore the accumulated knowledge of our scientific and regulatory heritage in a bid to declare nanosilver materials as new chemicals, with unknown properties and automatically harmful simply on the basis of a change in nomenclature to the term “nano”.
Introduction
Antimicrobial Biocides
History of Nanosilver Production and Use
Registration of Nanosilver Products in the United States
| category | conclusion | selection basis | number of registrations |
|---|---|---|---|
| confirmed nano | nano | evaluation based on public citations stating nano nature of product or direct measurements on product material (e.g., Figures 3 and 4) | 7 (7%) |
| likely nano | nano | evaluation based on patent literature and/or manufacturing techniques | 42 (46%) |
| ionic | not nano | products contain materials that store and release individual silver ions | 31 (34%) |
| not likely nano | not nano | products contain macro scale materials, e.g., silver-coated fibers | 8 (9%) |
| unknown | not nano | insufficient information available | 4 (4%) |
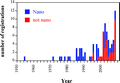
Figure 2. Registration of biocidal silver and nanosilver products in the U.S. with the categorization according to Table 1.
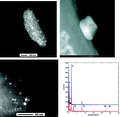
Figure 3. TEM analysis of silver-impregnated carbon filter, Zodiac Nature2 G (EPA registration no. 67712-1). Top left: larger particle with silver particles, discernible by their brightness; top right: magnification of one silver nanoparticle from the picture to the left; bottom left: small silver nanoparticles on gray matrix; bottom right: EDX spectra of the two areas in the TEM picture on the left (1: silver particles, bottom spectrum, 2: background: top spectrum). For details about methods see Supporting Information.

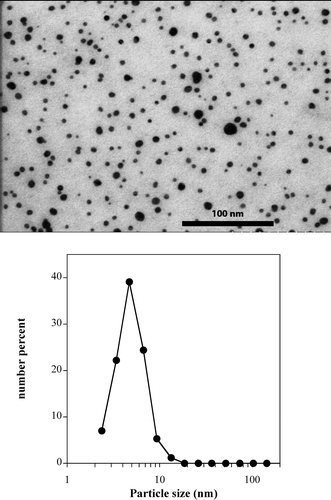
Figure 4. TEM analysis of the algicide Algaedyn (EPA registration no. 68161-1) (top) and particle size distribution by image analysis (bottom). For details about methods see Supporting Information.
| product type | product name | company | EPA registration number | date registered | reference |
|---|---|---|---|---|---|
| water filter | 989 Bacteriostatic Water Filter Media | Barnebey & Sutcliffe Corp (Now owned by Calgon) | 58295-1 | 1-Dec-1988 | a |
| water filter | NATURE2 G45-VC40 | Zodiac Pool Care, Inc. | 67712-1 | 21-Nov-2002 | b, Figure 3 |
| algicide | Algaedyn | Pool Products Packaging Corp | 68161-1 | 31-Dec-1954 | Figure 4 |
| algicide | Nu-Clo Silvercide | Alden Leeds Inc. | 7124-101 | 15-Jun-1993 | d |
| algicide | ASAP-AGX | American Biotech Laboratories | 73499-1 | 27-Feb-2002 | c |
| additive | Additive SSB | Nanohorizons Inc. | 83587-3 | 28-Sep-2007 | d |
| additive | MicroSilver BG-R | Bio-Gate AG | 84146-1 | 18-Mar-2008 | e |
| additive | HyGate 4000 | BASF Corp (Formerly Ciba) | 70404-10 | 5-Sep-2008 | e |
US Patent 3,374,608, “Silver Impregnated Carbon” (1968), Pittsburgh Activated Carbon Company, now owned by Calgon (“activated carbon impregnated with a metallic silver having a crystallite size of not over 250 A [25 nm].”...“[T]he silver must be dispersed as particles of colloidal size (less than 250 A”) (emphasis added).
US Patent 6,165,358, “Water Purifier for a Spa” (2000). Zodiac Pool Care, Inc. “purification materials are described, for example, in U.S. Pat. No. 5,352,369”. US Patent 5,352,369, “Method of Treating Water” (1994). Fountainhead Technologies Inc.: “the elemental silver preferably includes at least 2% of silver crystals having crystal sizes between approximately 3 nm and 10 nm” (emphasis added).
“These engineered silver particles currently vary in size between about 10−50 nm in diameter”. (April 26, 2005) William D. Moeller, President, American Biotech Laboratories Testimony on Malaria before the U.S. House of Representatives, International Relations Committee, Subcommittee on Africa, Global Human Rights, and International Operations. [http://www.foreignaffairs.house.gov/archives/109/20915.pdf] (p.38)].
SNWG “Evaluation of Hazard and Exposure Associated with Nanosilver and Other Nanometal Oxide Pesticide Products”, Presentation to Scientific Advisory Panel (November 4, 2009). Docket ID: EPA-HQ-OPP-2009-0683-0165. [http://www.regulations.gov/search/Regs/contentStreamer?objectId=0900006480a52512&disposition=attachment&contentType=pdf].
Ciba Specialty Chemicals (Now BASF) “Ciba Specialty Chemicals forms marketing cooperation with Bio-Gate for silver antimicrobial technology” (December 14, 2005, Basel, Switzerland) [http://cibasc.com/index/med-index.htm?reference=41794&checkSum=C44184952B5155A13ECC5E419C8F7310] Note figure caption “Scanning electron microscopy showing the high porosity HyGate 4000 powder: primary particle size 50−200 nm...” (emphasis added).
Silver Nanotoxicology
Implications for Policy of Nanosilver
Acknowledgment
We thank Dr. James Delattre and Dr. Rosalind Volpe of the Silver Nanotechnology Working Group (SNWG) for valuable contributions to the background of this article. We thank Dr. Ralf Kaegi from Eawag for performing the TEM analyses.
TEM analysis of water filters and TEM analysis of Algaedyn. This material is available free of charge via the Internet at http://pubs.acs.org.
References
This article references 47 other publications.
- 2.Wiesner, M. R.; Lowry, G. V.; Jones, K. L.; Hochella, M. F.; Di Giulio, R. T.; Casman, E.; Bernhardt, E. S.Decreasing Uncertainties in Assessing Environmental Exposure, Risk, and Ecological Implications of Nanomaterials Environ. Sci. Technol. 2009, 43 ( 17) 6458– 6462
- 3.Wijnhoven, S. W. P.; Peijnenburg, W. J. G. M.; Herberts, C. A.; Hagens, W. I.; Oomen, A. G.; Heugens, E. H. W.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; Dekkers, S.; De Jong, W. H.; van Zijverden, M.; Sips, A. J. A. M.; Geertsma, R. E.Nano-silver - A review of available data and knowledge gaps in human and environmental risk assessment Nanotoxicology 2009, 3 ( 2) 109– 138
- 4.Scheringer, M.; MacLeod, M.; Behra, T.; Sigg, L.; Hungerbühler, K.Environmental risks associated with nanoparticulate silver used as biocide Household Pers. Care Today 2010, 1, 34– 37
- 6.Benn, T. M.; Westerhoff, P.Nanoparticle silver released into water from commercially available sock fabrics Environ. Sci. Technol. 2008, 42 ( 11) 4133– 4139
- 7.Geranio, L.; Heuberger, M.; Nowack, B.Behavior of silver nano-textiles during washing Environ. Sci. Technol. 2009, 43, 8113– 8118
- 8.Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R.Toxicity of Silver Nanoparticles to Chlamydomonas reinhardtii Environ. Sci. Technol. 2008, 42 ( 23) 8959– 8964
- 10.Bell, F. A.Review of effects of silver-impregnated carbon filters on microbial water quality J. Am. Water Works Assoc. 1991, 83 ( 8) 74– 76[CAS]
- 14.Aruguete, D. M.; Hochella, M. F.Bacteria-nanoparticle interactions and their environmental implications Environ. Chem. 2009, 7 ( 1) 3– 9[CrossRef]
- 15.EPA. Nanotechnology White Paper; Report EPA 100/B-07/001; U.S. Environmental Protection Agency: Washington, DC, 2007.
- 16.Gottschalk, F.; Scholz, R. W.; Nowack, B.Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles Environ. Modeling Software 2010, 25, 320– 332[CrossRef]
- 17.Lea, M. C.On allotropic forms of silver Am. J. Sci. 1889, 37, 476– 491
- 19.Henglein, A.; Giersig, M.Formation of colloidal silver nanoparticles: Capping action of citrate J. Phys. Chem. B 1999, 103 ( 44) 9533– 9539[ACS Full Text
 ], [CAS]
], [CAS] - 20.Dong, X. Y.; Ji, X. H.; Wu, H. L.; Zhao, L. L.; Li, J.; Yang, W. S.Shape Control of Silver Nanoparticles by Stepwise Citrate Reduction J. Phys. Chem. C 2009, 113 ( 16) 6573– 6576[ACS Full Text
 ], [CAS]
], [CAS] - 22.Boese, K.Über Collargol, seine Anwendung und seine Erfolge in der Chirurgie und Gynäkologie Dtsch. Z. Chir. 1921, 163 ( 1−2) 62– 84
- 23.Bogdanchikova, N. E.; Kurbatov, A. V.; Tret’yakov, V. V.; Rodionov, P. P.Activity of colloidal silver preparations towards smallpox virus Pharm. Chem. J. 1992, 26 ( 9−10) 778– 779
- 24.Bechhold, H.Die Gallertfiltration Z. Chem. Ind. Kolloide 1907, 2, 3−9– 33−41
- 25.Moudry, Z. V.Process of producing oligodynamic metal biocides. United States Patent 2,927,052, 1953.
- 26.Manes, M.Silver impregnated carbon. United States Patent 3,374,608, 1968.
- 28.Kasemo, B.; Johansson, S.; Persson, H.; Thormählen, P.; Zhdanov, V. P.Catalysis in the nm-regime: Manufacturing of supported model catalysts and theoretical studies of the reaction kinetics Top. Catal. 2000, 13, 45– 53[CrossRef]
- 29.Ryu, S. K.; Eom, S. Y.; Cho, T. H.; Edie, D. D.Distribution of Silver Particles in Silver-containing Activated Carbon Fibers Carbon Sci. 2003, 4 ( 4) 168– 174
- 31.Heinig, C. F.Method of treating water. United States Patent 5,352,369, 1994.
- 32.Schlee, H.; Zweifel, E.Über das Verhalten von Silberpräparaten, insbesondere von Kollargol im Organismus Z. Hyg. Infekt. 1924, 102, 454– 460[CrossRef]
- 34.Gaul, L. E.; Staud, A. H.Seventy cases of generalized argyria following organic and colloidal silver medication, including biospectrometric analysis of ten cases J. Am. Med. Assoc. 1935, 104 ( 16) 1387– 1390
- 36.Hill, W. R.; Pillsbury, D. M. Argyria, the Pharmacology of Silver; The Williams & Wilkins Co.: Baltimore, MD, 1939.
- 38.EPA. Registration eligibility document Silver, List D, Case 4082; U.S. Environmental Protection Agency: Washington, DC, 1992.
- 40.Lee, K. J.; Nallathamby, P. D.; Browning, L. M.; Osgood, C. J.; Xu, X. H. N.In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos ACS Nano 2007, 1 ( 2) 133– 143
- 41.Choi, O.; Hu, Z. Q.Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria Environ. Sci. Technol. 2008, 42 ( 12) 4583– 4588
- 46.Kim, B.; Park, C.-S.; Murayama, M.; Hochella, M. F.Discovery and Characterization of Silver Sulfide Nanoparticles in Final Sewage Sludge Products Environ. Sci. Technol. 2010, 44, 7509– 7514
- 47.Gottschalk, F.; Sonderer, T.; Scholz, R. W.; Nowack, B.Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions Environ. Sci. Technol. 2009, 43, 9216– 9222
Citing Articles
Citation data is made available by participants in CrossRef's Cited-by Linking service. For a more comprehensive list of citations to this article, users are encouraged to perform a search in SciFinder.
This article has been cited by 11 ACS Journal articles (5 most recent appear below).
Degradation Products from Consumer Nanocomposites: A Case Study on Quantum Dot Lighting
Jingyu Liu, John Katahara, Guanglai Li, Seth Coe-Sullivan, and Robert H. HurtEnvironmental Science & Technology2012 46 (6), 3220-3227Degradation Products from Consumer Nanocomposites: A Case Study on Quantum Dot Lighting
Jingyu Liu, John Katahara, Guanglai Li, Seth Coe-Sullivan, and Robert H. HurtEnvironmental Science & Technology2012 46 (6), 3220-3227Most nanomaterials enter the natural environment as nanoenabled products, which are typically composites with primary nanoparticles bound on substrates or embedded in liquid or solid matrices. The environmental risks associated with these products are ...
Characterization of Silver Nanoparticle Products Using Asymmetric Flow Field Flow Fractionation with a Multidetector Approach – a Comparison to Transmission Electron Microscopy and Batch Dynamic Light Scattering
H. Hagendorfer, R. Kaegi, M. Parlinska, B. Sinnet, C. Ludwig, and A. UlrichAnalytical Chemistry2012 84 (6), 2678-2685Characterization of Silver Nanoparticle Products Using Asymmetric Flow Field Flow Fractionation with a Multidetector Approach – a Comparison to Transmission Electron Microscopy and Batch Dynamic Light Scattering
H. Hagendorfer, R. Kaegi, M. Parlinska, B. Sinnet, C. Ludwig, and A. UlrichAnalytical Chemistry2012 84 (6), 2678-2685Due to the already prevalent and increasing use of silver-nanoparticle (Ag-NP) products and the raised concerns in particular for the aquatic environment, analytical techniques for the characterization of such products are of need. However, because Ag-NP ...
Size-Controlled Dissolution of Organic-Coated Silver Nanoparticles
Rui Ma, Clément Levard, Stella M. Marinakos, Yingwen Cheng, Jie Liu, F. Marc Michel, Gordon E. Brown, Jr., and Gregory V. LowryEnvironmental Science & Technology2012 46 (2), 752-759Size-Controlled Dissolution of Organic-Coated Silver Nanoparticles
Rui Ma, Clément Levard, Stella M. Marinakos, Yingwen Cheng, Jie Liu, F. Marc Michel, Gordon E. Brown, Jr., and Gregory V. LowryEnvironmental Science & Technology2012 46 (2), 752-759The solubility of Ag NPs can affect their toxicity and persistence in the environment. We measured the solubility of organic-coated silver nanoparticles (Ag NPs) having particle diameters ranging from 5 to 80 nm that were synthesized using various methods,...
- alt="Cover Image" class="cover" width="115" height="153">
Silver Nanoparticles and Total Aerosols Emitted by Nanotechnology-Related Consumer Spray Products
Marina E. Quadros and Linsey C. MarrEnvironmental Science & Technology2011 45 (24), 10713-10719Silver Nanoparticles and Total Aerosols Emitted by Nanotechnology-Related Consumer Spray Products
Marina E. Quadros and Linsey C. MarrEnvironmental Science & Technology2011 45 (24), 10713-10719Products containing silver nanoparticles are entering the market rapidly, but little is known about the potential for inhalation exposure to nanosilver. The objectives of this work were to characterize the emissions of airborne particles from consumer ...
Generation of Metal Nanoparticles from Silver and Copper Objects: Nanoparticle Dynamics on Surfaces and Potential Sources of Nanoparticles in the Environment
Richard D. Glover, John M. Miller, and James E. HutchisonACS Nano2011 5 (11), 8950-8957Generation of Metal Nanoparticles from Silver and Copper Objects: Nanoparticle Dynamics on Surfaces and Potential Sources of Nanoparticles in the Environment
Richard D. Glover, John M. Miller, and James E. HutchisonACS Nano2011 5 (11), 8950-8957The use of silver nanoparticles (AgNPs) in antimicrobial applications, including a wide range of consumer goods and apparel, has attracted attention because of the unknown health and environmental risks associated with these emerging materials. Of ...
Tools
-
 Add to Favorites
Add to Favorites
-
 Download Citation
Download Citation
-
 Email a Colleague
Email a Colleague -
 Permalink
Permalink
 Order Reprints
Order Reprints Rights & Permissions
Rights & Permissions Citation Alerts
Citation Alerts
History
- Published In Issue February 15, 2011
- Article ASAPJanuary 10, 2011
- Received: September 30, 2010
Accepted: December 16, 2010
Revised: December 13, 2010


 ACS
Network
ACS
Network